Product
VDx® AIV qRT-PCR products
Avian influenza Real-time RT-PCR products
Introduction
VDx® AIV qRT-PCR products are used for detection and identification of viral RNA of AIV by real-time PCR method.
Feature
- Confirmatory diagnosis of AIV
- Suitable for screening of AIV outbreak
- High sensitivity and specificity
| Intended use | Detection and identification of Avian influenzavirus (AIV) RNA |
|---|---|
| Specimens | Stool, tissue homogenates (lung, spleen, tonsil), Oral swab, Virus culture |
| Specimens | Poultry (chicken or duck, etc.) |
| Method | Real-time RT-PCR, qRT-PCR |
Technical Data
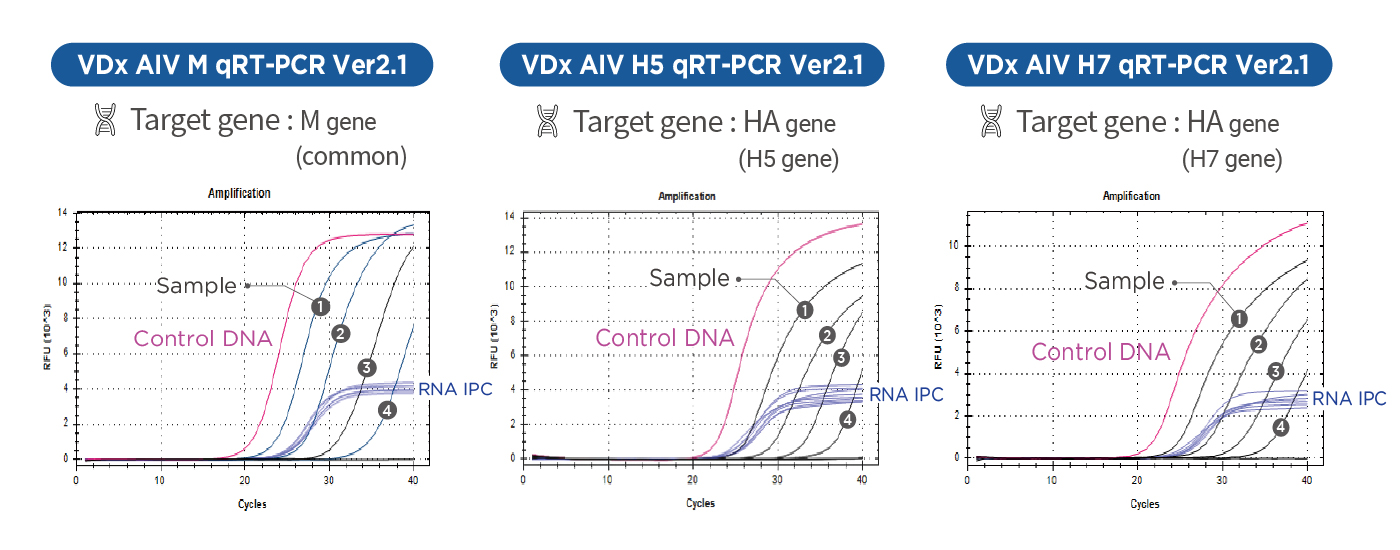
AIV Total Diagnosis System
Product information
| Cat No. | Product name | Packing unit | Manual |
|---|---|---|---|
| NP-AIV-38 | VDx® AIV M qRT-PCR Ver 2.1 | 96 Tests/Box |
|
| NP-AIV-39 | VDx® AIV H5 qRT-PCR Ver 2.1 | 96 Tests/Box |
|
| NP-AIV-3A | VDx® AIV H7 qRT-PCR Ver 2.1 | 96 Tests/Box |
|
| NP-AIV-34 | VDx® AIV H9 qRT-PCR (Research Use Only, RUO) | 96 Tests/Box |
|